|
APPLICATION NOTE |
|
|
Gas Chromatography - Mass Spectrometry lipid biomarker analysis and balance equation solutions for determination the genera-species composition of microbial communities |
GENITAL INFECTIONS AND STD |
Looking for microbial markers in body fluids.
Fatty acid composition and microbial community in urine, vaginal fluid and semen as detected by gas chromatography - mass spectrometry.
| Gas chromatography - mass spectrometry in microbe detection. Theory |
Unfortunately, currently available methods are not sufficiently rapid and universal for slow growing bacteria, anaerobes, nonfermenters and other extraordinary microbes. Chemical methods currently finding increased use to distinguish micro-organisms in applied research turn to be far more rapid [1,2]. The two most widely used methods are gas chromatography (GC) and gas chromatography-mass spectrometry (GC-MS), which are able to provide extensive information on monomer chemical components of microbial cells and metabolites [3]. Such cellular chemical components (or markers) can be detected among other chemical constituents of any biological object, indicating the presence of the corresponding microbial genus or species in the community under study [4-6]. Different applications of chemical marker method were surveyed by Morgan [7]. Chemodifferentiation is, of course, widely used as a method of determining and verifying the taxonomic position of micro-organisms. It is applied for identification of micro-organisms isolated in pure cultures and is based on the use of very large databases containing data on the fatty acids composition of a few thousand strains of bacteria and microscopic fungi. The Microbial Identification System (MIS), marketed by MIDI Inc., Delaware [8], is an example of such systems. Fatty acid features are commonly used now in bacterial taxonomy and clinical bacterial diagnostics [9]. In our approach, we use both available and new methods of identification and enumeration of micro-organisms in native samples, and these methods do not require prior growing or isolating colonies on selective media. The local databases we developed for different ecological niches store our own data and those found in literature on the chemical composition of micro-organisms inhabiting this particular niche. Such a constraint eliminates the ambiguity in species identification inherent in the use of general data banks given that the fatty acids compositions of some micro-organisms from different ecosystems are very close.
Consistent, standardised methods to measure and quantify microbial chemical markers directly in body fluids have not been established. Nevertheless, early, prompt and accurate diagnosis is of importance and prospective for improving strategy of antimicrobial therapy.
| Previous investigations |
Only a few research has been undertaken for clinical diagnostic by using marker method. Well-known is gas chromatographic method for diagnosis of candidiasis by arabinitol detection in serum and urine [10]. Muramic acid content in blood was used for nonspecific detection of bacteria [11]. Other study include the discovery of gram-negative bacteria by its lipopolysaccharide (LPS) specific hydroxy fatty acids [12]. Furthermore, 3-hydroxymyristic acid have been identified in biological fluids as marker of Haemophylus [13,14], and also were used to differentiate Campylobacter species [15]. Meningococci were followed in blood by measuring 3-hydroxylauric acid content using GC-MS single ion monitoring technique [16]. Neisseria gonorrhoae have been followed in blood and other samples by using the same method [17]. Honococcal infection were estimated if h12/h14>0.2 (the proportion of 3-hydroxylauric to 3-hydroxymyristic acid).
| Our approach |
This approach was broadened to simultaneous determination of more than one bacteria by its lipid markers and profiles as a result of whole biomass lipid content analysis. Fatty acid concentrations were considered in balance equations with partial include of single micro-organisms proposed as community members. Computerised solution appears to be effective in discovery of genera-species composition of some ecological microbial communities [18,19,20]. Furthermore, this method have been transformed to detection symbiotic and invasive microflora and follow-up infection and disbiosis in body fluids with their 104 background lipid prevalence [21,22].
The present work was undertaken to systematically study of lipid markers and associated clinically important microbes of urogenital tract (UGT). Such experimental study did not have an analogues in science periodic and therefore need for convincing statements that each chemical marker used are of microbial origin and refer to a specific micro-organism. The lack of direct cross-validation of this approach and traditional microbiological, biochemical and genetic methods complicates its evaluation. Really, it seems to be complicated to apply various methodology to one sample in order to get information simultaneously about aerobic, anaerobic bacteria, intracellular parasites, mycobacteria, nonfermenters, etc. Nevertheless we have confirmation of markers in statistical behaviour of biological fluid lipid patterns and the detected microflora and also in patient history, clinical data and therapy effectiveness. Here we report the results both analytical review of modern considerations of urogenital microflora (mostly vaginal) and specific lipid patterns of their members. We also report preliminary results of mixed infection and disbiosis analysis in more than 500 samples of urine, vaginal fluid and semen of gynaecologist and urologist patients. The analysis is patent supported [18, 22,23], certified by medical authority [24], and have been done by request of physician and with patient accordance.
Analytical assay and database for urogenital microbial community diagnostic by using chemical markers
| Vaginal microflora features |
Vaginal microbial community include normal microflora, facultative and real pathogens amd also sexual transmitted disease (STD) agents. Normal microflora represented by Lactobacillus, Bifidobacterium, Corinebacterum, Staphylococcus epidermidis [25]. Vaginal community of healthy woman have been shown to consist of 108cells aerobes and 109 anaerobes per 1 g of vaginal fluid as measured by culture method [26]. More frequently isolated are Lactobacillus, Peptococcus, Bacteroides, S.epidermidis, Corinebacterium sp., Peptostreptococcus sp., Eubacterium. Putrid bacteria content such as Bacillus, Proteus, Clostridium, Spirohaeta depends upon vagina purity. They are usually differentiated from purulent ones, associated with local or generalised infections caused by pathogenic staphylococci, streptococci, Serratia, Enterobacter, Flavobacterium meningosepticum, Neisseria meningitidis. Microscopic fungi are usually found [25] and also Peptostreptococcus as associated with Bacteroides and Trichomonas [27]. Candida, Chlamidia, Trichomonas, Mycoplasma associated with N.gonorrhoae were usually isolated from venereal clinic patients. C.trachomatis is commonly found in young and T.vaginalis in elder woman [28]. Corinebacterium vaginale, yeasts, Mycoplasma, Staphylococcus and diphteroids are common for girls. N.gonorrhoae, Trichomonas, Chlamidia, Lactobacillus are isolated in elder ones and enterobacteria in junior [29].
| Microbes in semen |
Little research has been undertaken to analyse microflora in human semen. E.coli, Staphylococcus, Pseudomonas, Klebsiella, Enterobacter, Bacillus, Neisseria Corinebacterium, Micrococcus, Proteus, Achromobacter and microscopic fungi were isolated from infertile males [30]. Aerobic and anaerobic bacteria, Mycoplasma, Herpes simplex virus, yeasts and filamentous fungi were found in male genital ulcerations. M.hominis, M.urealyticum and M.genitalium are their usual inhabitant. Mycoplasmas are isolated sometimes at prostatitis and infertile semen [31, 32].
| Specific cell lipid components of bacteria. Confirmation of markers |
This part presents a detailed description of cell specific lipid patterns, i.e. fatty acids, hydroxy fatty acids, which are proposed as lipid markers for detection and follow-up urogenital infection and disbiosis. They have been measured in pure cultures of micro-organism by experts in microbial fatty acids profiles, i.e. C.W.Moss, E.Yantzen, I.Brondz, E.Miyagawa and many others. References wil be presented in special scientific paper. This markers are of general value in human due to partial similarity of the microflora of various organs.
Lactobacillus. Lactobacillus, or Dederlein rods are common inhabitant of female genital organs. They have lactobacillic acid (19cyc) as marker of genera in urogenital community. Of course this acid may be specific for another bacteria. Apart the ecological ones there are two groups of micro-organisms in man having this feature. There are Pseudomonas and Enterobacteriaceae members. However this bacteria were not frequently identified species from both urea and genital samples, and their include in chromatographic peak area of lactobacillic acid can be enumerated by using fatty acid balance equations.
Chlamidia trachomatis. Many of the microorganisms of this ecological nishe has strong lipid markers. One of them is Chlamidia trachomatis, having three hydroxy acids of marker value: 3-hydroxyeicosanoic (3h20), 3-hydroxyisoeicosanoic (3hi20) and 3-hydroxydocosanoic (3h22) acids. They form a strong collective marker for Chlamidia.
Mycobacterium. Tuberculostearic acid (TSA) or 10-methyloctadecanoic acid (10Me18) is a well-known marker for diverse group of mycolate-containing bacteria, i.e. genera Mycobacterium, Corinebacterium, Nocardia. However none of them were detected in urogenital organs during ordinary surveys. C.urealyticum, C.seminale commonly found in this niche but they occasionally did not posses TSA. Nocardia not reported in connections with urogenital microflora. Consequently, TSA refer to Mycobacteria in this case as nonspecific marker. Nevetheless, Mycobacterium sp. have been shown to contain species-specific long-chain 3-hydroxy fatty acids and specific fatty alcohols [Jantzen, Larsson, Liquin]. As a result 3-OH-2,4,6-tri-Me-tetracosanoic acid was determined as additional marker for M.tuberculosis.
Eubacterium. It has been known that Eubacterium methabolize human cholesterol to coprostanol (dehydrocholesterol). Eubacterium is one of the of the main component of interstinal microflora. Coprostanol is widely used as a marker of fecal contamination in environment.
Corinebacterium urealyticum. Mycobacterial cell wall of C.urealyticum have been shown to contain specific polyunsaturated fatty acids with hydroxyl group in third position and long-chain (C10-C16) hydrocarbon branching at second carbon atom (mycolic acid). A total of six different acids were found in C.urealyticum. However, C30:3 and C28:1 are of analytical significance. The remain part of FA composition in C.urealyticum is identical with main host cell FA.
Klebsiella. Earlier 2-hydroxymyristic acid (2h14) has been detected in K.pneumoniae and confirmed in our clinical isolates. This substance appear to be genera-specific marker of Klebsiella in community under study.
Peptostreptococcus anaerobius. This micro-organism have been shown to have a distinct marker - isomyristic acid (i14).
Bacillus. This genera is characterised by branch-chain fatty acid prevalence. It is noteworthy that i16 (iso-hexadecanoic) and a13 (anteisotridecanoic) acids present in bacillus, but not in other bacteria of urogenital community. Consequently we use this substances to follow-up bacilli.
Pseudomonas. Two hydroxy acids, 3-hydroxydecanoic and 3-hydroxydodecanoic are known as a markers of genera. First of them is preferable for investigated community, as far as second one interfere with such of Neisseria.
Pseudomonas aeruginosa. This species can be differentiated by 2-hydroxydodecanoic acid which is of marker value in this community.
Clostridium perfringens. In an earlier study we identified two methabolites of C.perfringens groop (C.perfringens, C.histolyticum and C.novii) associated with anaerobic clostridial infections and resulting probably in interaction of bacteria with host cells. This is 10-hydroxystearic (10h18) and 10-hydroxyoctadecenoic (10h18:1) acids. C.perfringens as well as Eubacterium represent the main microflora of intestine. This unusual hydroxy acids are widely spread in environment with faecal contaminants and detectable in all human and animal organs, except skin.
Clostridium sp. Group of clostridia including C.fallax, C.sordelly have substantial quantity of tetradecenoic (14:1) acid in cell FA profile, and we used it to watch this group in our samples.
Bacteroides fragilis. This bacterium and also B.fragilis group members are characterised by 3-hydroxyisoheptadecanoic acid as a main hydroxy acid in cell envelope LPS.
Streptococcus. Most of streptococci are invisible in lipid extracts because of similarity of fatty acid s composition with biological fluid background. Nevertheless oral streptococci group (S.mutans, S.salivarius and so on) can be detected in specimens by presence of decanoic acid which is absent in healthy patient. Enterococci S.faecium, S.faecalis can be detected, if present, by lactobacillic acid, which is largely of minor value in real urogenital samples. Streptococcus pneumonia has tetradecenoic (14:1) acid in cell FA profile and interfere with some clostridia.
Candida albicans. Specific feature of this microscopic fungi in lipid moiety is heptadecenoic acid (17:1) which found only in C.albicans.
Microscopic fungi. Non-specific marker for microscopic fungi of clinical value, such as Aspergillus, Candida, Mucor and other, is ergosterol.
Flavobacterium. This organism was found as a causative agent of inflammation in newborn and originally transmitted from mother. F.meningosepticum pointed out as a purulent bacteria in vagina. So we include isoheptadecenoic acid (i17:1) as a marker of genera in our assay. Beside this flavobacterium FA profile include unusual 2-hydroxy acids, namely 2-hydroxyisopentadecanoic (2hi15) and 2-hydroxyisogeptadecanoic (2hi17) which also have a marker value in biological fluids of human being.
Streptomyces. This is one of the most probable micro-organisms, as well as Micromonospora, and Bacillus associated with plenty of isohexadecanoic (i16) acid in body fluid lipid preparations. This actinomycetes are rare organisms which posses branched FA with even number of carbon atoms. Streptomyces was earlier isolated in our clinic and found to contain about 14% of i16 acid in whole FA profile. Streptomyces was being found also as endocarditis causative agent.
The remaining part of community members seems to be lack of markers and interfere with one or more bacteria in the same substance.
Neisseria gonorrhoeae. N.gonorrhoeae is commonly found in venerologist patients. This species is characterised by high content of 3-hydroxylauric acid (3h12) which content is 10-20% in total FA profile and 3-hydroxymyristic (3h14) acid. This acids are part of LPS in Neisseria and also in Pseudomonas, Moraxella and Acinetobacter species. The interference must been solved in balance equations. Fortunately, alternative bacteria are usually absent in real samples and all response in 3h12 can be devoted to N.gonorrhoeae. I.Sud empirically estimated that positive diagnosis can be estimated when 3h12/3h14>0,2.
Bacteroides urealyticum. This microbe is probably a single one associated with 3-hydroxyhexadecanoic acid (3h16) in body fluids. Of course there are another bacteria (Brucella, Francisella, Pseudomonas putida) having 3h16 among cell FA, but first two organisms are strong pathogens of epidemiological value, and P.putida is commonly and preferably found in ecological communities. Occationally, Helicobacter pylori also possess 3h16 in cell envelope and must be taken into account when assigning GC-MS response to 3-hydroxy-hexadecanoic acid.
Fusobacterium and Haemophylus. The only distinctive compound in cell wall of this micro-organisms is 3-hydroxy-myristic acid which unfortunately interfere with many others having 3h14 in FA profile. Such is E.Coli, Klebsiella, Serratia and others. The solution of balance equations is needed if this bacteria are present in sample simultaneously.
Corinebacterium-Listeria group. This group is suspected in any samples due to unfitted quantity of anteiso-heptadecanoic (a17) acid after balance equation solution. GC-MS response in a17 is much more than predicted by content of known bacteria listed in database. So we include this group in database as far as only one having a17 as the main (up to 50%) in FA profile.
Staphylococcus. Staphylococci were found to contain various branched fatty acids (i15, a15, i17, a17, a19). The differences between three clinically important species i.e. S.aureus, S.haemolyticus and S.epidermidis are significant and species can be separated mathematically.
| Analytical procedure |
Patients and Methods.
Patients. Gynaecologist and urologist patients asked to participate in GC-MS investigations as additional test in complicated cases. They are from local ambulance and Central Institute of Dermatology and Venerology, Moscow, Russia. Patients were sampled during routine urogenital examinations. Samples of vaginal content, semen or urine immediately transported to laboratory or applied to conservation in methanol or frizzed up to analysis.
Sample preparation.
Body fluid (5ml urea, 0,5ml semen or 0.1ml vaginal fluid) were subjected to lipid extraction procedure in chloroform-methanol mixture followed by acid methanolysis of dry lipid residue in 0,2ml dried HCl in methanol 2,5N by heating to 80oC for 1hr. Phospholipid fatty acid methyl esters (FAME), lipopolysaccharide (LPS) hydroxy FAME were formed as a result and extracted with hexane. The hexane fraction were dried, and dry residue was sylilated in 20mkl N,O-bis-trimethylsilyl-trifluoroacetamide (BSTFA) by heating at for 15min.
Gas chromatography- mass spectrometry (GC-MS) analysis.
Measurements were performed on a Hewlett-Packard HP-5973 MSD, or Shimadzu QP-2000 systems with a cross-linked methyl silicone capillary column (Ultra-1). The oven temperature was 2 min at 120oC and then programmed to 320oC at 5 grad/min. 1-2mkl of derivatized sample were injected in gas chromatograph at 2800C. Fatty acids and other lipid components after separation in GC column were ionised by electron impact method and analysed in the selected ion monitoring (SIM) mode using appropriate ions for thirty microbial markers distributed in five separate ion groups throughout analytical range from decanoic acid to heaviest cholesterol metabolites. For instance, ion 87 was used for nonhydroxy and ion 175 for hydroxy FA. Each substance was confirmed by another specific ion, molecular or fragment, which is characteristic in mass spectrum. There was M-15 ion for 3-OH-acids and M-59 ions for 2-OH-acids. Molecular ion and M-32 ion were used for confirmation saturated and monounsaturated fatty acid. Another confirmations of markers were achieved by measuring the specific retention times and ratio of chromatographic peak areas for selected ions of single marker substance. The analysis of lipid moiety by GC-MS-SIM technique provides a convenient way of obtaining quantitative data in spite of surpassing background caused by fluid and epithelial cells components. This is because we did not include in SIM and time programme the strongest ions of bulk liquid. Known quantity of hydroxy fatty acids or known number of cells was examined in separate experiment for calibration. Heptadecanoic acid was used as internal standard (body fluid invariant) for comparative calculations.
Quantitative determination of the species composition.
Methodology and mathematical treatment for quantitative determination of the species composition of microbial communities from GC-MS data were published earlier [20]. Briefly, the following m linear simultaneous equations in n unknowns can be written for m biomass components and n bacterial genera (species), where i is subscript for the lipid components of the total biomass and j of the micro-organisms.
A1. q = X1R1,1 + X2R1,2 + X3 R1,3 +.........+ XnR1,n
A2. q = X1R2,1 + X2R2,2 + X3R2,3 +.........+ XnR2,n
A3. q = X1R3,1 + X2R3,2 + X3R3,3 +.........+ XnR3,n (1)
::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::
Am. q = X1Rm,1 + X2Rm,2 + X3Rm,3 +.........+ XnRm,n
where Ai (i = 1, 2, ..., m) is the peak area of the ith compound in the chromatogram of the total biomass, Xj (j = 1, 2, ..., n) is the number of cells of the jth micro-organism, q is the scale coefficient depending on analysis conditions and instrument sensitivity, and Rij is the content of the ith compound in the fatty acid profile of the jth micro-organism. This equation set has a unique solution provided m = n and equations are independent, that is none of them can be expressed as a linear combination of other equations.
The solution of equation set (1) is given by
Xj = Dj/D, (2)
where Dj is the determinant of a partial matrix of coefficients for the jth component and D is the determinant of equation set (1). The number of equations in this set can be as large as the number of components of the cellular fatty acid pool determined by GC-MS. There is information on as many as 150 lipid components in our data bank. At the same time, micro-organisms contains only 10 to 20 compounds from this list, meaning that most compounds from this set are not present in any individual micro-organism. This, in turn, means that most of the coefficients Rij are zero and the system is, therefore, degenerate and can be reduced to several subsystems of smaller rank (and fewer unknowns). Given that the number of members of actual microbial communities studied is between 30 and 40 and the number of fatty acid that can be detected in the total biomass of any given community is 70 to 80, we can select 30 to 40 equations such that the rank of the obtained subsystems be minimal. Finally, it should be taken into consideration that, within the community studied, many microbial constituents have the status of biomarkers, i.e., are specific to only one taxon of micro-organisms. Such micro-organisms can be enumerated from the concentration of their marker, Xi = Aiq/Rij. This makes it possible to eliminate the corresponding unknowns and to reduce the rank of the entire equation set.
The value of the coefficient q in the latter relationship can be calculated as follows
q = (V/(vm sample))(102/(Qm0))/S = 1/(q1q2S).
In this relationship, q1 = msample v/V accounts for the share of the sample injected into the chromatograph, and q2 = Qm0/102 expresses the mass of fatty acid constituents of single cell of micro-organism. Assuming that 1 g of bacteria contains 5.9*1012 cells and each cell contains, on average, 3% of lipid monomers (fatty acids and aldehydes), q2 = 3/(5.9*1014) = 5.08*10-15 g. S can be found as Astand/mstand, where Astand is the peak area of a standard employed (a compound with a predetermined concentration) and mstand is the amount of the standard used in instrument calibration.
| EXAMPLES |
The presence of different bacteria, protozoa, and intracellular parasites in urogenital organs complicates the study of disbiosis by traditional methods. Gas chromatography-mass spectrometry was used for identification and quantitative estimation of micro-organisms by the presence of chemical compound or microbial metabolites in vaginal fluid urine and semen.
The two most widely used methods are gas chromatography (GC) and gas chromatography-mass spectrometry (GC-MS), which are able to provide extensive information on monomer chemical components of microbial cells and metabolites [3]. Such cellular chemical components (or markers) can be detected among other chemical constituents of any biological object, indicating the presence of the corresponding microbial genus or species in the community under study [4-6].
This short-term analysis (5 hours) is universal, does not require precultivation and pure cultures isolation using selective media. Test biological specimens not needed. Local database and programme of marker control was developed for GCMS research based on lipid biomarker analysis and balance equation methodology and PC calculation with the Microsoft Excel Tables.
Trace quantity of chemical markers analysed directly in biological fluid which are in contact with microbes and contain viable and disrupted cells and their metabolites. The presence of branched and odd acids, hydroxy acids and certain aldehydes is specify for bacteria. The dynamic of alterations of fatty acids composition pointed to their exogenic origin up to the rate of 0.001% total pool of fatty acids in biological liquids. Data treatment of 70 samples by the ranging method showed the resemblance to the frequency of occurrence of certain micro-organisms described in literature. Statistical treatment the data of more than hundred patient show two separate clusters to each marker substance, and consequently to corresponding micro-organism. One of them refer to normal concentration in healthy state, and another associated with it pathogenic activity.
The results of investigation genera-species composition of vaginal fluid by Gas Chromatography - Mass Spectrometry method..
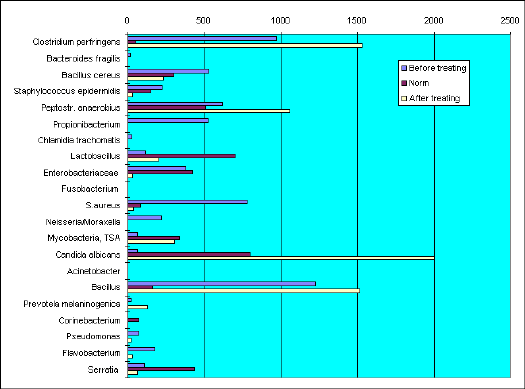
Sample VF-417. Measurements before and after treating.

BEFORE TREATING
Microflora composition differ against normal in excess of Bacillus, Staphylococcus aureus, and also occurance of Neisseria gonorrhoae, Pseudomonas, Flavobacterium and Chlamidia trachomatis. Content of lactobacilli is very low.
AFTER TREATING
As compared to previous analysis quantity of Chlamidia, Propionibacterium and Neisseria were eradicated. S.aureus, S.epidermidis, Flavobacterium, Pseudomonas and enterobacteria decreased tenfold. As a results of specific treating against Neisseria and Chlamydia concurrent microscopic fungi Candida, Peptostreptococcus, Clostridium perfringens, mycobacteria and Bacillus became prevalent.
Rationalised treating towards concurrent microflora moved microbial community to normal level.
Candida albicans and associated infection
Gas Chromatography - Mass Spectrometry investigation of vaginal microflora genera-species composition.
Sample VF-962. T-a.
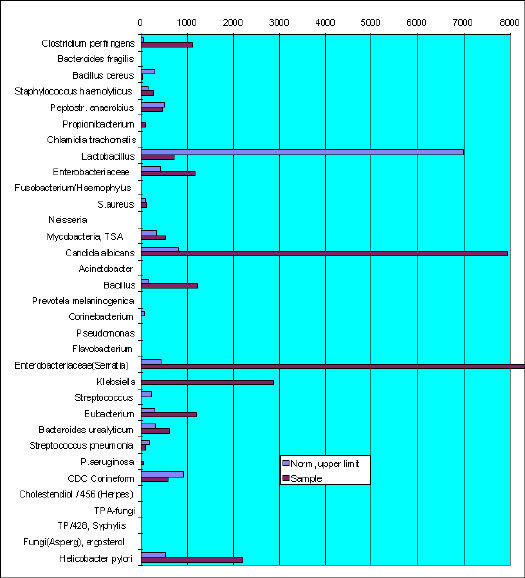
The content of Candida albicans marker (17:1, heptadecenoic acid) overestimate normal level substantially. As well as markes of (10h18, 10-hydroxystearic acid), members of Enterobacteriaceae family (18:1D 11, vaccenic acid), Klebsiella (2h14, 2-hydroxymiristic acid). Eubacterium and Helicobacter pylory are in excess of norm.
Physician could take in account Candida albicans and Clostridium perfringens simultaneous occurrence. Both micro-organisms cause the same clinical feature of candidiasis.
Infectious agent changing
Gas Chromatography - Mass Spectrometry investigation of vaginal microflora genera-species composition.
Sample VF-786. I-a.
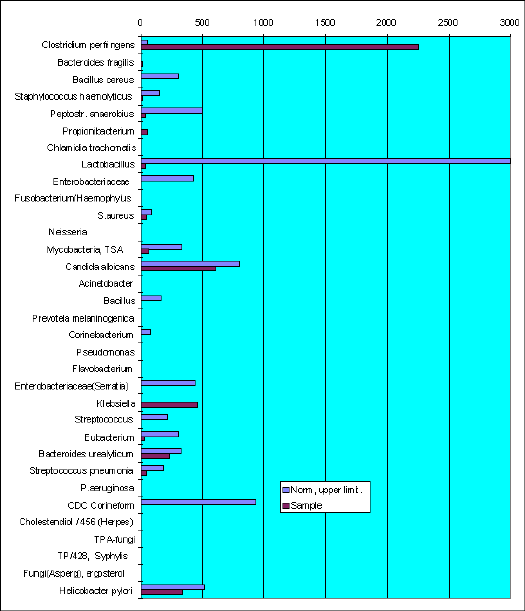
The leading microflora are presented by Ńlīstridium perfringens and Klebsiella This case is common when Candida albicans, usual causative agent have changed by Ńlīstridium perfringens. Antifungal chemicals administered to patient did not effective in such cases.
C.perfringens produce volatile fatty acids, which foaming vaginal fluid and make it white like that of candidiasis patients.
Helicobacter pylori in vagina?
Gas Chromatography - Mass Spectrometry investigation of vaginal microflora genera-species composition.
Sample VF-772. A-n.
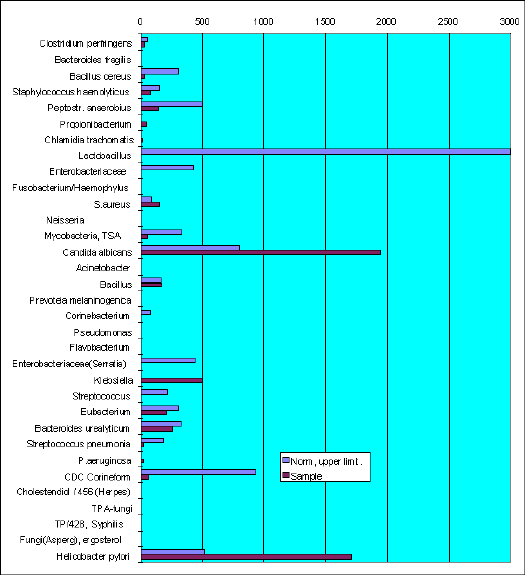
There are chlamidia markers found in the sample.
The leading microflora was presented by Candida albicans and Helicobacter pylory.
|
?? |
Question to experts. If anybody have found Helicobacter pylori in female genitales? |
This micro-organism is known as stomach and duodenum ulcer causative agent. It never found in other human organs. Probably due to H.pylory diagnostic difficulties. GC-MS have preferences in detection that microbe by its specific marker - hydroxyoctadecanoic acid, which did not produced by another micro-organisms of clinical value and human cells.
Honococcal and anaerobic infection
Gas Chromatography - Mass Spectrometry investigation of vaginal microflora genera-species composition.
Sample VF-953. Z-a.
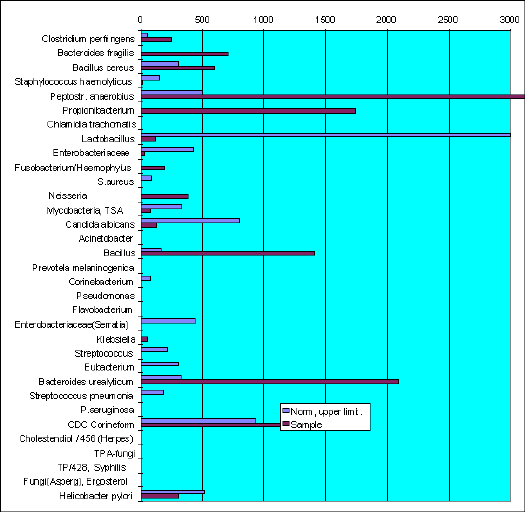
Hydroxydodecanoic and hydroxytetradecanoic acids - markers of Neisseria honorrhoae - was found in the sample.
Anaerobic Clostridium perfringens, Bacteroides fragilis, B.urealyticum, Fusobacterium, Peptostreptococcus anaerobius and Propionibacterium are the micro-organisms which markers exceed the normal level in vaginal content of healthy women.
References